Products found to contain undeclared medicines
|
| |
| Woman arrested for suspected illegal sale of slimming products with banned drug ingredient on the Internet (with photo) |
| |
A 63-year-old woman was arrested today (July 11) in a joint operation by the Department of Health (DH) and the Police for suspected illegal sale and possession of Part I poisons and unregistered pharmaceutical products. The products concerned were suspected to contain banned drug ingredients and other Western medicines.
Upon the DH's investigation of a public complaint, samples of products claiming to be for slimming purposes were purchased via the Internet for analysis. Test results showed that four products contained Western medicines. One of them contained sibutramine and hydrochlorothiazide, while the remaining three contained fluoxetine, chlorpheniramine and bisacodyl respectively. The seller alleged that the slimming products were obtained from Thailand. She was arrested by the Police in the operation today.
The DH's investigation is continuing.
"Sibutramine is a Part I poison which was once used as an appetite suppressant. Since November 2010, products containing sibutramine have been banned because of an increased cardiovascular risk. Hydrochlorothiazide is a diuretic used for the treatment of hypertension and it may cause hypotension and electrolyte imbalance. Fluoxetine is used for depression and may cause diahorrea and insomnia. Hydrochlorothiazide and fluoxetine are Part I poisons which should only be supplied at pharmacies under the supervision of a registered pharmacist upon a doctor's prescription. Bisacodyl is an over-the-counter laxative that may cause abdominal pain. Chlorpheniramine is an over-the-counter antihistamine which may cause drowsiness," a DH spokesman explained.
According to the Pharmacy and Poisons Ordinance (Cap 138), all pharmaceutical products must be registered with the Pharmacy and Poisons Board of Hong Kong (the Board) before they can be legally sold in the market. Illegal sale and possession of Part I poisons and unregistered pharmaceutical products are criminal offences. The maximum penalty for each offence is a fine of $100,000 and two years' imprisonment.
The spokesman strongly urged members of the public not to buy or consume slimming products of unknown or doubtful composition or from unknown sources. Those who have purchased such products should stop taking them immediately and consult healthcare professionals if they are in doubt or feeling unwell. All registered pharmaceutical products should carry a Hong Kong registration number on the package in the format of "HK-XXXXX". Unregistered pharmaceutical products have not been evaluated by the Board and their safety, quality and efficacy may not be guaranteed.
"Weight control should be achieved through a balanced diet and appropriate exercise. The public should consult healthcare professionals before using any medication for weight control," the spokesman advised.
Ends/Thursday, July 11, 2013
Issued at HKT 20:11
NNNN
|
| |
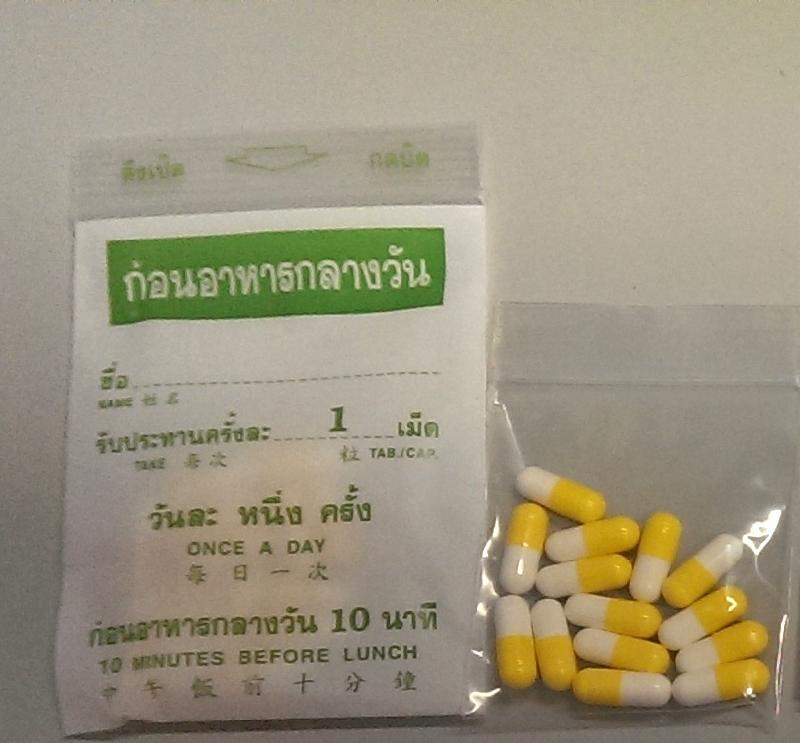 | | Test results showed that one of the slimming products allegedly obtained from Thailand contained sibutramine and hydrochlorothiazide. | | |
|



